Molarity, Molality, Normality, Part per million (ppm)and other basic termof Concentration solution along definition & formula |Chemistry Basic|02
This is article about basic of chemistry in glance and brief were considered in the Question & answer format. This will be helpful to the student who want to ace in practical viva exams and written exams. It is also helpful for preparation of interview. So, folks let commenced and learn just by glance on it:
[Caution.: The aim of this article focus on the student and Job hunters to get right information at right time in unceratain and dilemma situation along with distraction of Social media and Binge. For others such as layman can also learn at their own risk. ]
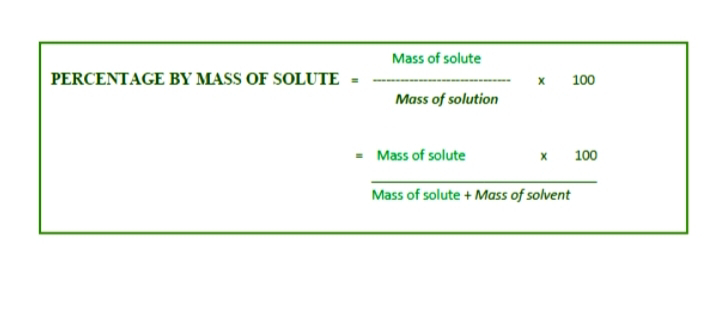
What is Percentage of mass (W/W)?
The mass of solute in gram dissolved in solvent to form 100 gram of solution is called Percentage of mass.
What is Percentage by volume (V/V)?
It is defined as the ratio of the number of part by the solute to one hundred parts of the solution.
What is Mole Fraction?
Mole fraction of a component in a solution is defined as the ratio of number of moles of that component present in the solution to that total number of moles of all component of the solution.
What is Molarity?
It is defined as the number of moles of solute present in 1 L volume of the solution.
What is Molality?
It is defined as the number of mole of solutes dissolved in 1 Kg or 1000 g of solvent.
What is Formality?
One formal solution (1F) is defined as the solution that contains one formula wt. of the solute in 1L of the solution.
What is Normality?
Normality (N) of a solution is defined as the number of equivalents of the solute present in (1 L) of the solution.
What is Part Per Million (ppm)?
• The number of parts of one component in 1 million parts of a solution.
• The concentration of solute present in trace quantity is conveniently expressed in part per million (ppm).
What is Part Per Billion (ppb)?
• The number of parts of one component in 1 billion parts of a solution.
• This unit is lower concentration than ppm is extensively used to express concentration of pollutants or the amount of the trace material.
If you like this article please follow this website. Share this to your friends and adorable folks. Share your precious feedback in the comment section. Your feedback and support will be appreciated.








Comments
Post a Comment