Concentration of Solution and Terminology of Concentration solution-01: Chemistry Basic part. :01

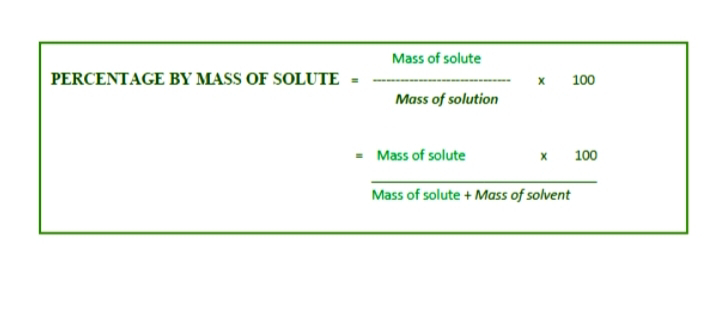
This is article about basic of chemistry in glance and brief were considered in the Question & answer format. This will be helpful to the student who want to ace in practical viva exams and written exams. It is also helpful for preparation of interview. So, folks let commenced and learn just by glance on it: [Caution.: The aim of this article focus on the student and Job hunters to get right information at right time in unceratain and dilemma situation along with distraction of Social media and Binge. For others such as layman can also learn at their own risk. ] What is concentration of solution? The concentration of solution is defined as the amount of solute dissolved in a specific amount of solvent. What is Dilute solution? Solution containing relatively less amount of solute are called Dilute solution. What is Concentrated solution? Solution containing more amount of solute then the solution is called Concentrated solution What is Saturated solution? It contains less amount of