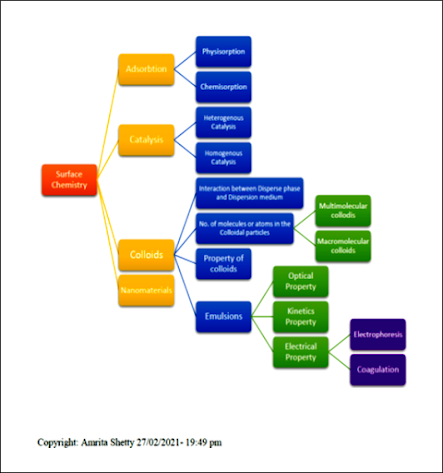
SURFACE CHEMISTRY
SURFACE CHEMISTRY
02/03/2021, Author.: Amrita Shetty
In this article, the Surface
chemistry information is took from Reference text book of state level. Where it
is made in simple Question& Answer and several chart flow is used for your mind
to get this content interactive and for the good grasping. I hope this will be
useful to understand this topic in simple way.
1.
What is
Adsorption? Adsorption
is, defined as the phenomenon of accumulation of higher concentration of one
substance on the surface of another than in the bulk. 2. What is Adsorbate or Adsorbed phase? Substance
adsorb in surface of another substance (Adsorbent) is called Adsorbate or
Adsorbed phase 3. What is Adsorbent? Substance which adsorb substance on its surface is called the Adsorbent. 4. What is process of Adsorbtion? Adsorption is exothermic process 5. What is Heat of adsorption? Heat evolved or released per mole of adsorbate is called Heat of adsorption. 6. What is Absorption, to distinguish Adsorption? Absorption is defined as the phenomenon of uniform distribution of one substance throughout the body of another substance. 7. What is example of Absorption? i. H2O vapour are absorb by the Calcium chloride to form Hydrate. ii. Ammonia is absorbed by H2O. 8. Where the H2O and Ammonia is adsorb? i. H2O adsorb by the Silica Gel. ii. Ammonia is adsorb by Charcoal. 9. What is Absorbent? The substance which absorbs another substance on its surface is called as Absorbent. 10. What is Absorbate? The substance which is absorb on the surface of another substance is called Absorbate. Or The substance which is absorbed by another substance is called Absorbate. |
ADSORPTION
Type of Adsorption
The type of adsorption
depend on the type of force involved in the adsorption.
PHYSISORPTION/ VAN DER WAALS
ADSORPTION/ PHYSICAL ADSORPTION
1. What is Physical adsorption?
The force between the particle of adsorbate and adsorbent are Physical in nature is called as Physical adsorption.
2. What is the name of forces for the Physical
Adsorption?
The force is known as Van der Waals forces.
3. What is Van der Waals forces?
This forces causes the condensation of gas into liquid.
4. What is magnitude of Physisorption?
The heat released in Physisorption is same as magnitude of heat of condensation of gases.
5. Why in magnitude of Physisorption heat
released is same as that of heat for condensation of gases?
Heat released in the Physisorption
is same magnitude as the heat released in the condensation of gases because the
Van der Waal forces is the weak forces.
At high temperature, the adsorbed
gases form several layer of molecule after adsorption.
6. When the Physisorption is large?
At low temperature, the adsorption extent is large.
7. When the Physisorption is low?
The adsorption is low at high
temperature.
Equilibrium in Van der Waals
adsorption is reached rapidly and is reversible.
8. What is the Condition required for
Physisorption?
The Physisorption is favourable at
high pressure or at low temperature of surface.
9. What is reverse condition for
Physisorption?
The Physisorption is reversed at
low pressure or high temperature of surface.
10. What is example of Physisorption?
i i. The various gases adsorb on the Charcoal. ii. The Oxalic acid solution adsorb by Charcoal. iii. The Acetic acid solution adsorb by Charcoal.
ii. T
CHEMICAL ADSORPTION/ CHEMISORPTION/
ACTIVATED ADSORPTION
1. Which Scientist
considered the chemisorption?
Chemisoprtion is considered by Irving Langmuir, American Chemist in 1916.
2. What is nature of
bond between the adsorbate and adsorbent?
The adorbate particle is connected to the surface of the adsorbent by chemical bond of the same type as the Covalent bond of particle in a molecule.
3. What is Covalent bond?
The
attractive force which exists due to the mutual sharing of electrons between
two atoms of similar electronegativity or having small difference in
electronegativites is called a Covalent bond.
4. What is process of Chemisorption?
Usually,
Chemisorption is Exothermic process.
5. When Chemsiorption is endothermic in
nature?
Chemisorption
in endothermic process is exceptional because in this process adsorbate
dissociate from the surface of adsorbent.
6. What are the example of Chemisorption in
endothermic process?
The
Adorbate Hydrogen gas molecule on the surface of the Adsorbent glass is the
endothermic process because Hydrogen atom dissociate from the adsorbent.
7. What is magnitude of heat in Chemisorption?
In chemisorption, the heat released or evolved
in Chemsisorption per mole of Adsorbate is same in magnitude as the heat in
chemical bonding.
8. Why chemisorption is known as Activated
adsorption?
This
chemisorption need high activation energy, so it is known as Activated
Adsorption.
9. Why Chemisorption need high activation
energy?
The
chemisorption is slow process, so it need high activation energy.
In
this Chemisorption, the gas molecule with surface form surface compound.
The
bonding in the Chemisorption is so tight that the compound will not desorb.
10. What are the Example of Chemsisorption?
The
Carbon monoxide is form when atomic oxygen adsorbed on the Graphite.
11. What happen while heating on the Graphite?
After
heating the Graphite surface, the Carbon monoxide will desorb but not Atomic
oxygen on the graphite because in this chemisorption the bond between the
adsorbate and adsorbent is tight.
12. What happen, when oxygen gas adsorb on the
Tungsten?
Tungsten
adsorb the oxygen gas and form Tungsten trioxide while adsorption.
13. When the Surface of Tungsten is heated,
what occurs?
When
Tungsten surface is heated, the Tungsten trioxide will desorb but oxygen will
remain on the surface.
14. What is another example of Chemisorption?
i. O2 adsorb on the graphite surface.
ii. CO adsorb on the Tungsten.
iii. Oxygen adsorb on the Ag, Au, Pt & Tungsten.
iv. H2 adsorb on the Nickel.
15. Is there any adsorption which can act in
both Physical and chemical adsorption at which situation?
Many adsorption are neither
Physical nor chemical but have both combination. There are some adsorption
which is physical at low temperature and when the same adsorption is chemical
at high temperature.
16. When the adsorption become Physisorption
and Chemisorption?
At
low temperature the adsorption become Physisorption and when temperature is
raised the adsorption become Chemisorption.
17. What are the example which show both
Physisorption and Chemsisorption?
The H2 adsorb on the
Nickel show Physisorption at low temperature and Chemisorption at high
temperature.
REFERENCEI.:
11th Chemistry Text book of
Maharashtra State Board

Comments
Post a Comment